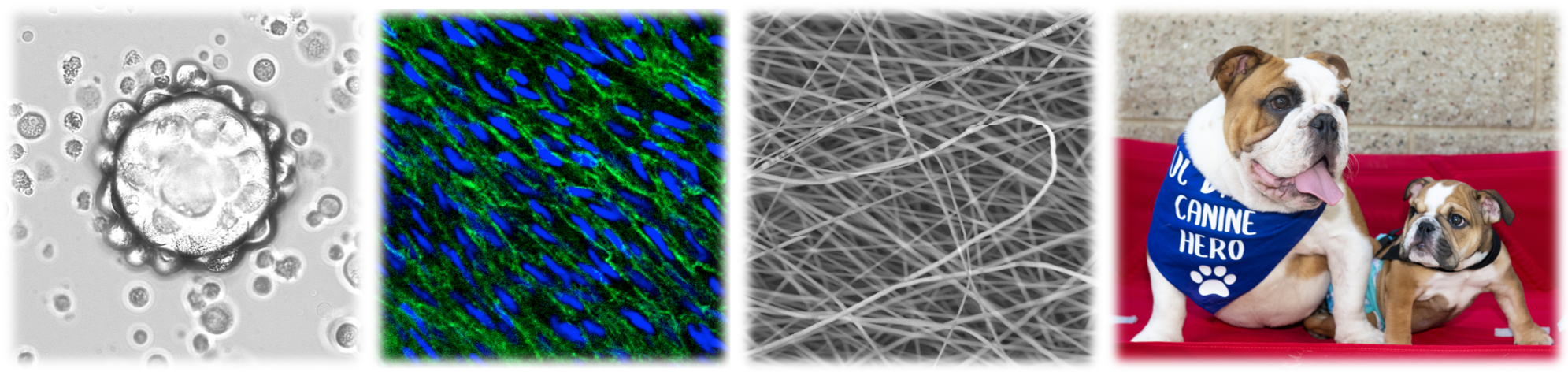
Our Research
Dr. Wang’s research program focuses on developing innovative tools, technologies, and therapeutics that combine molecular, cellular, tissue, and biomaterial engineering to promote regeneration and restore function. Dr. Wang’s lab engineers and develops products and technologies using stem cells, extracellular vesicles, lipid nanoparticles, and extracellular matrices to treat a wide range of medical conditions and diseases, and employ cutting-edge single-cell spatial omics technologies and AI to accelerate discoveries in precision medicine and targeted therapies. Current projects include:
(1) Studying stem cell biology, differentiation, and secretion
We derive stem cells from various sources and investigate the mechanisms of action behind stem cell benefits, their differentiation capabilities, and secretion profiles to improve their application as regenerative therapeutics for various diseases.
(2) Engineering stem cell-derived extracellular vesicles (EVs) for tissue regeneration and targeted delivery
Extracellular vesicles (EVs) mediate critical cell-to-cell communication and have emerged as a new class of nanotherapeutics for regenerative medicine. We develop high-density culture methods to improve yields of stem cell-derived EVs for translational applications. We engineer EVs to improve their therapeutic functions by loading chemical drugs or biological molecules, and engineering their surface to improve targeting efficiency.
(3) Developing gene editing strategies for treatment of genetic diseases
Novel gene editing approaches are transforming medicine. We develop novel extracellular vesicles, lipid nanoparticles (LNPs), and virus-like particles (VLPs) to overcome limitations such as limited stability, low endosomal disruption rates, and high toxicity. These approaches are non-viral, potentially less immunogenic alternatives to traditional virus-based methods. We are particularly interested in applying gene editing in utero to treat early diagnosable genetic diseases. By administering this therapy in utero, there is an opportunity to correct the genetic defect before the onset of the syndrome's manifestations, possibly preventing or significantly reducing the disease's impact on development.
(4) Applying single-cell spatial multi-omics technologies for enhanced diagnostics, prognosis, and therapies
Single-cell spatial multi-omics technologies have revolutionized our ability to analyze complex biological systems at unprecedented resolution, offering significant potential for enhanced diagnostics, prognosis, and therapies. Single-cell spatial multi-omics integrates various techniques to simultaneously analyze multiple molecular layers (e.g., genome, transcriptome, proteome, epigenome, and metabolome) while preserving spatial information within tissues. We routinely use cutting-edge spatial technologies such as CosMx and MALDI Mass Spectrometry Imaging in the lab. The rich, multi-dimensional data enables more sophisticated predictive models that can provide personalized prognostic information based on a patient's unique cellular and molecular profile and contribute to therapeutic advancements.
(5) Advancing AI-driven drug discovery and bioinformatics
Our research harnesses cutting-edge artificial intelligence to revolutionize drug discovery and bioinformatics analysis. We employ state-of-the-art technologies such as vision transformers to accelerate and enhance the accuracy of drug efficacy evaluation. Our team has pioneered innovative, cost-effective, high-throughput methodologies for assessing drug effectiveness. A prime example of our work is the development of SIC50, a groundbreaking label-free approach that combines vision transformer technology with Sobel-edge detection. This method enables us to determine a drug's inhibitory concentration (IC50) directly from phase-contrast images, streamlining the evaluation process. Furthermore, we leverage the power of generative AI and large language models to tackle the complexities of bioinformatics analysis. These advanced tools allow us to efficiently process and interpret vast, multifaceted datasets, including those generated by single-cell spatial multi-omics techniques. This approach significantly enhances our ability to extract meaningful insights from complex biological data, potentially accelerating discoveries in personalized medicine and targeted therapies.
(6) Establishing and using experimental and naturally-occurring disease models to evaluate regenerative products and treatments
Effective research largely depends on the development of successful animal models that can accurately portray the disease processes observed in humans. Our team has been successfully using well-established, surgically- and drug-induced small and large animal models to rigorously test treatment products. In addition, naturally-occurring models, which can more accurately exemplify disease processes are also being used. These models are extremely beneficial not only for humans but also for veterinary scientific advancement in treatment options. Currently, the Wang group is actively collaborating with the UC Davis Veterinary Medicine team and translating the innovative treatments developed in the lab to treat companion animal patients.
(7) Generating clinical-grade stem cell products and conducting human clinical trials
Our team has developed rigorous protocols for the production of stem cells under current Good Manufacturing Practice (cGMP) at the UC Davis GMP facility, and carried out extensive investigational new drug (IND)-enabling studies required by the U.S. Food and Drug Administration (FDA) for clinical applications. In particular, Dr. Wang has been collaborating with Dr. Diana Farmer for the past decade in developing a stem cell regenerative therapy for spina bifida and the team has recently received approval from the FDA to test a groundbreaking spina bifida treatment that combines surgery with stem cells. The one-of-a-kind treatment, delivered while the baby is still in the mother’s womb, could improve outcomes for children with the birth defect.
(8) Fostering academic innovation and entrepreneurship
Dr. Wang has established the Center for Surgical Bioengineering (CSB) as a hub for innovation and education, by integrating varying disciplines for a robust collaborative environment and by mentoring physicians, residents, scholars, and graduate and medical students to foster future innovation. The CSB is actively engaged with the emerging Aggie Square, the UC Davis - Sacramento Innovation Center. As the UC Davis School of Medicine Dean's Fellow in Entrepreneurship, Dr. Wang is devoted in expanding our institution’s infrastructure in innovation and entrepreneurship to better link advances in academic research with product and technology development and commercialization, and promoting UC Davis' contribution to socio-economic development.